Features and benefits
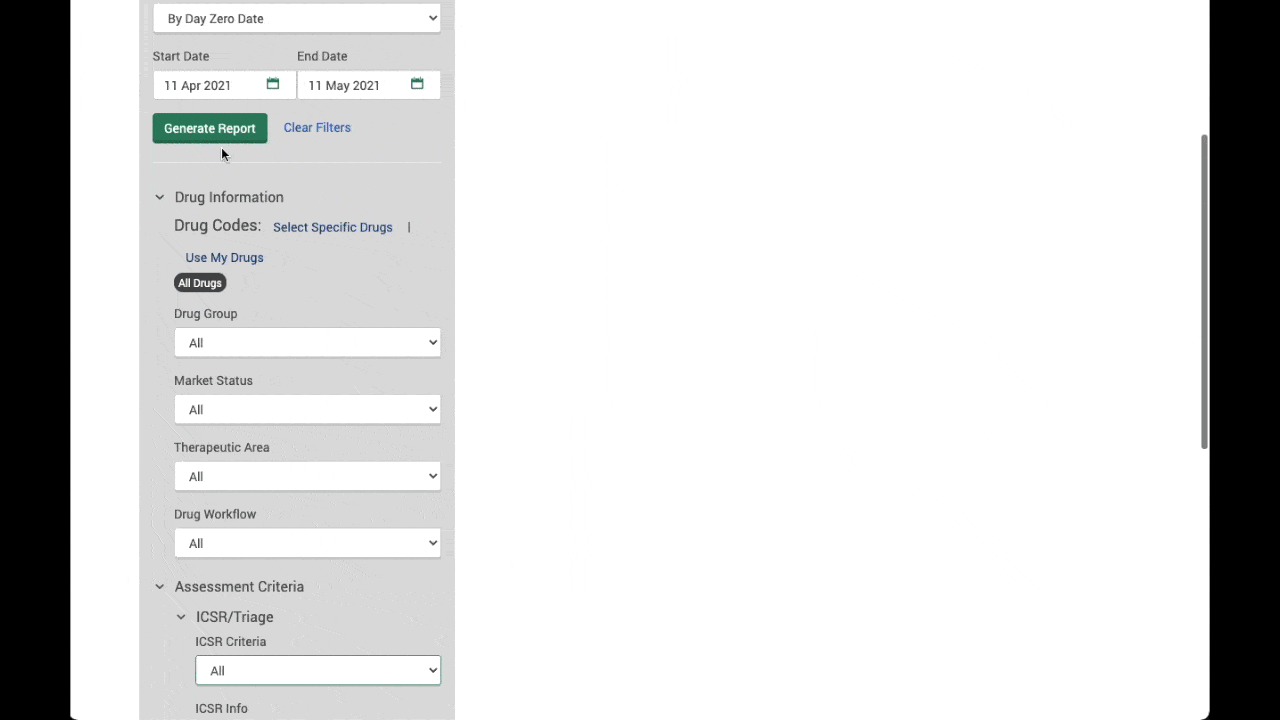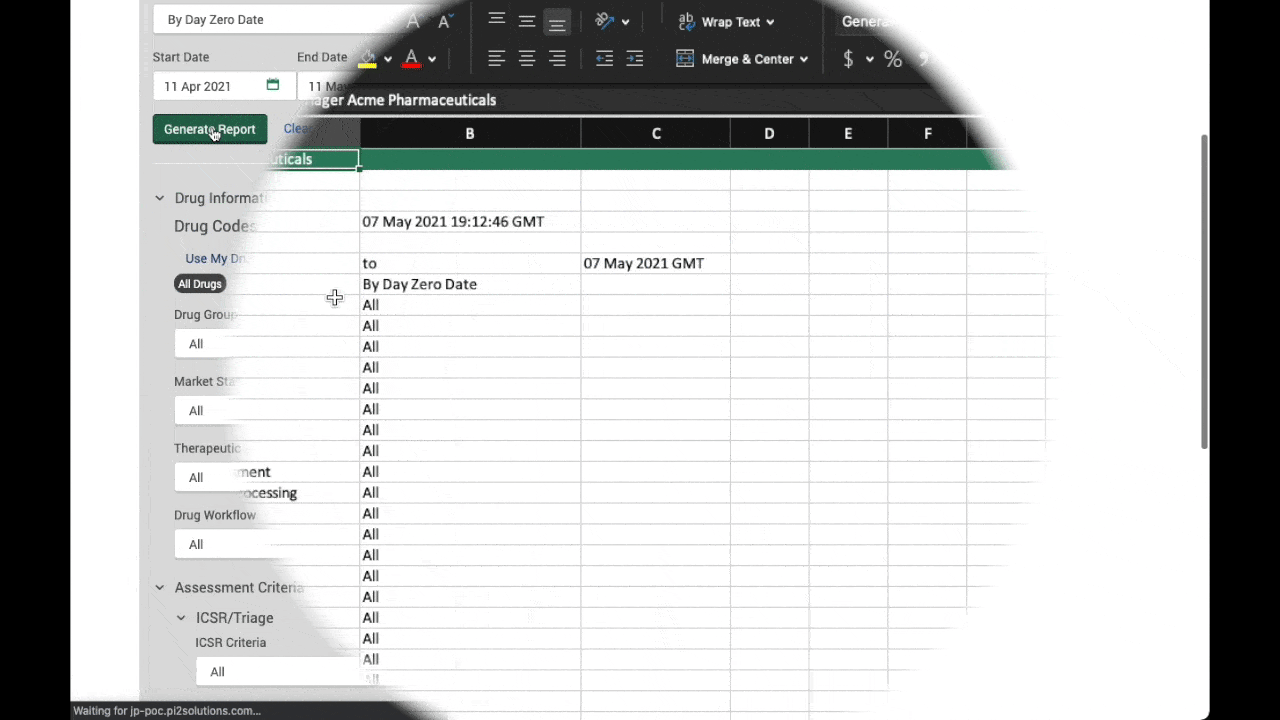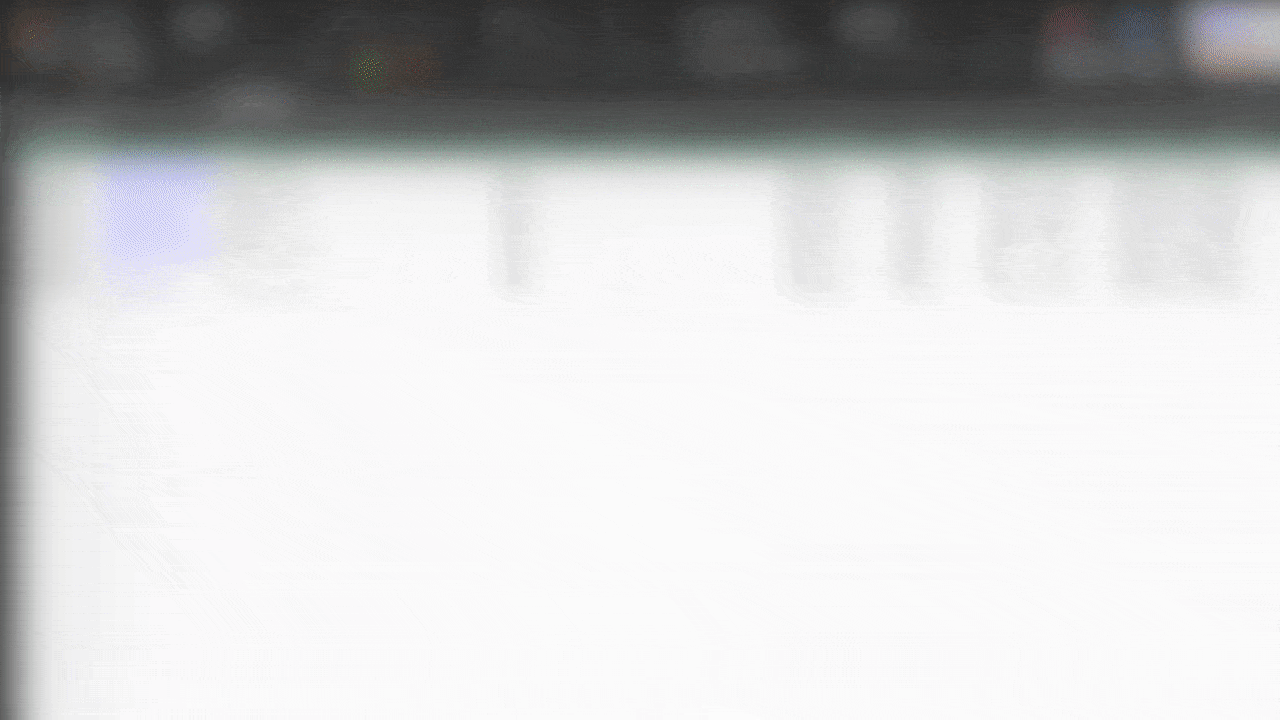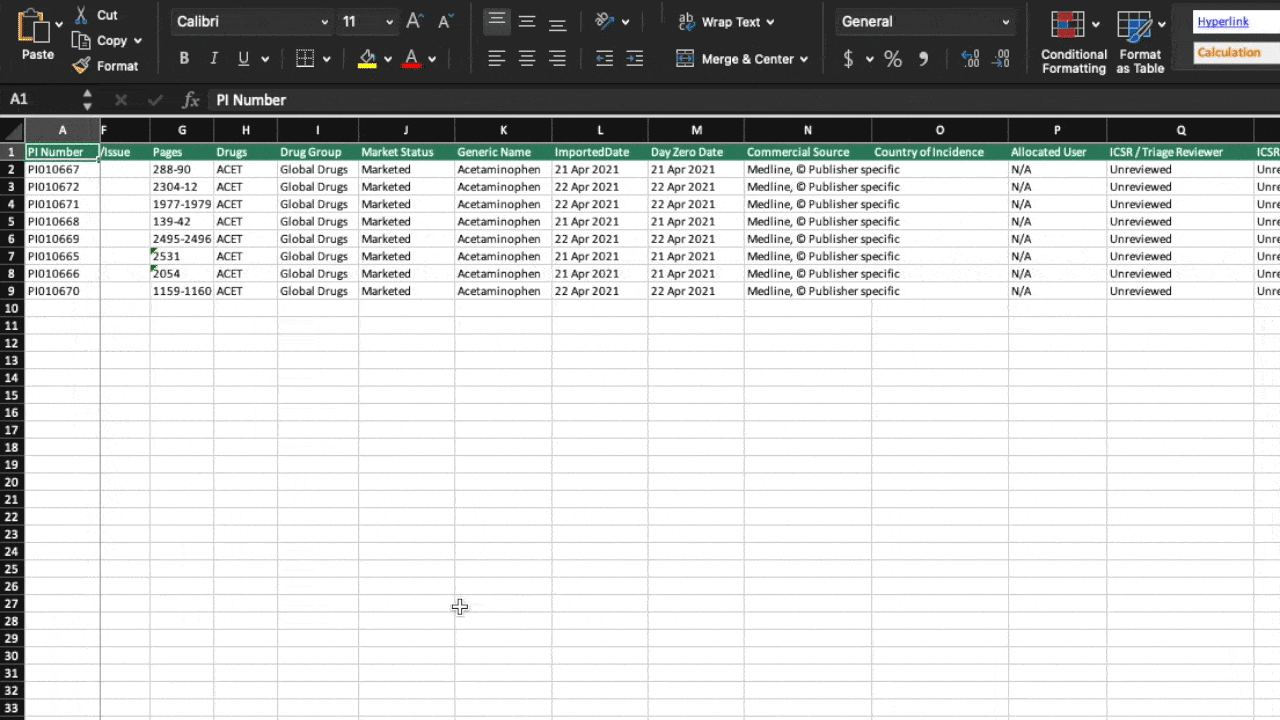
Configurable and easy to use
- Automatic import and work allocation means no reference is missed for review
- A modern and intuitive user interface allows users to concentrate on article analysis and assessment.
- Automatic audit-trail for each document
- Full text, translation and author follow-up support with day 0 change upon upload
- Workflows for global teams and global GVP regulations
- Timeliness tracking and quality control
- Compliant output: ICSR E2B R3 output to case processing, Aggregate Reports in Vancouver style and Safety Signals in line listing
- Extensive ad-hoc reports for proactive operation monitoring, work status visibility and reporting, oversight, and reconciliation.
Supported by artificial intelligence
Dialog Solution’s artificial intelligence engine DialogML is part of the Dialog platform. It uses Natural Language Processing to enhances Dialog alerts by automatically identifying references in scientific literature that meet the criteria for ICSR, Aggregate Reports or Safety Signals.
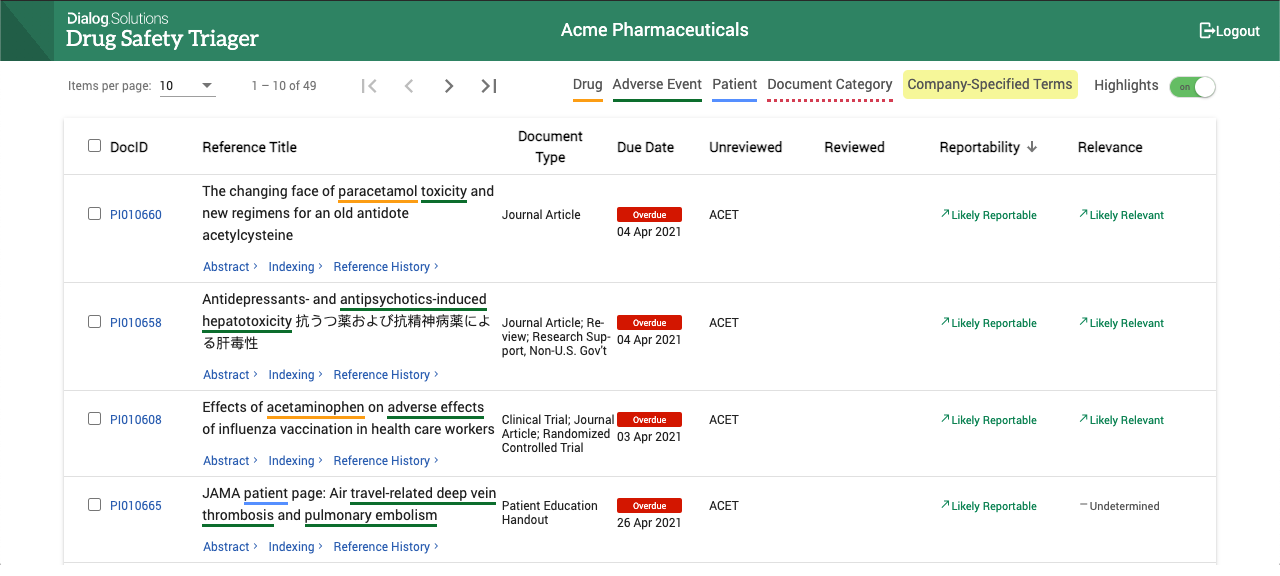
DialogML applies a patient safety relevancy ranking to each reference and adds drug safety tags. As a result, in the Drug Safety Triager the most relevant and time-sensitive references are presented at the front of the queue and have the drug safety relevant terms highlighted.
This allows review teams to better prioritise their workload, reduces the risk of late ICSR submissions and speeds up the review. With DialogML assistance, literature review teams can find ICSRs 5x faster and reduce the volume of literature to be reviewed for ICSRs by up to 50% without introducing additional risk.
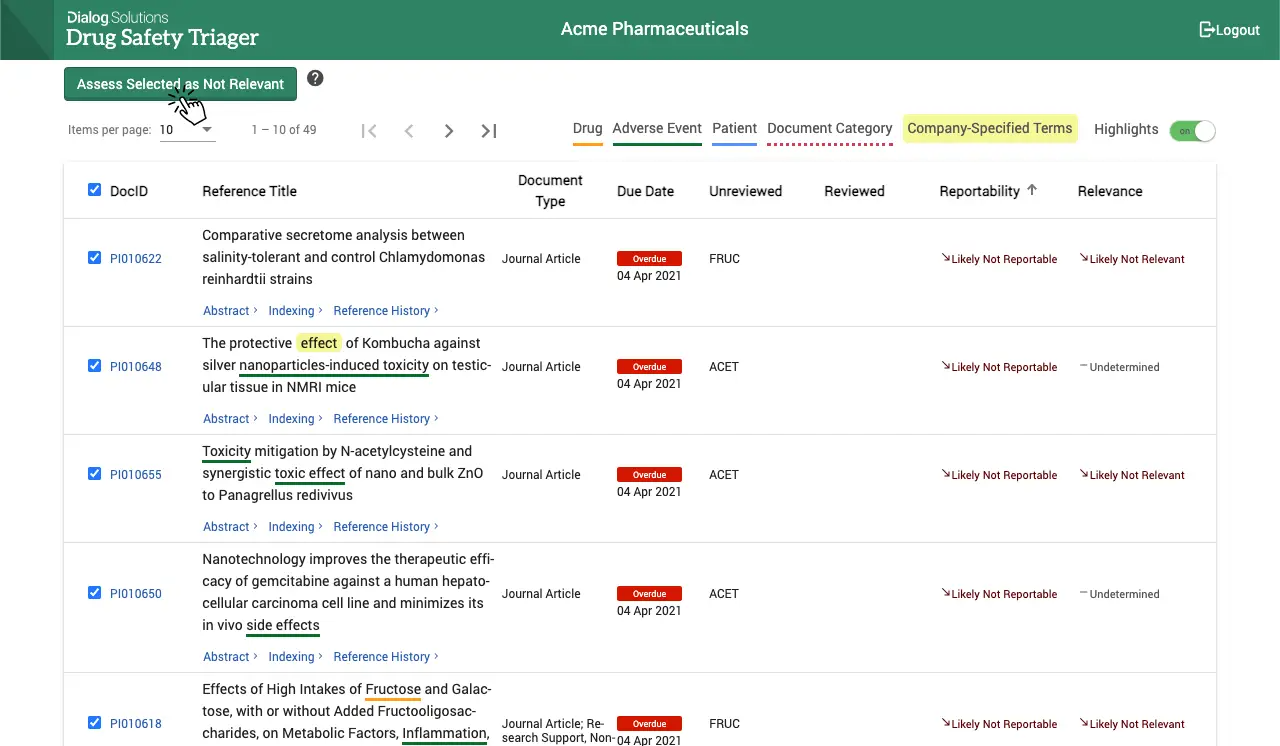
Increased efficiency with robotic process automation
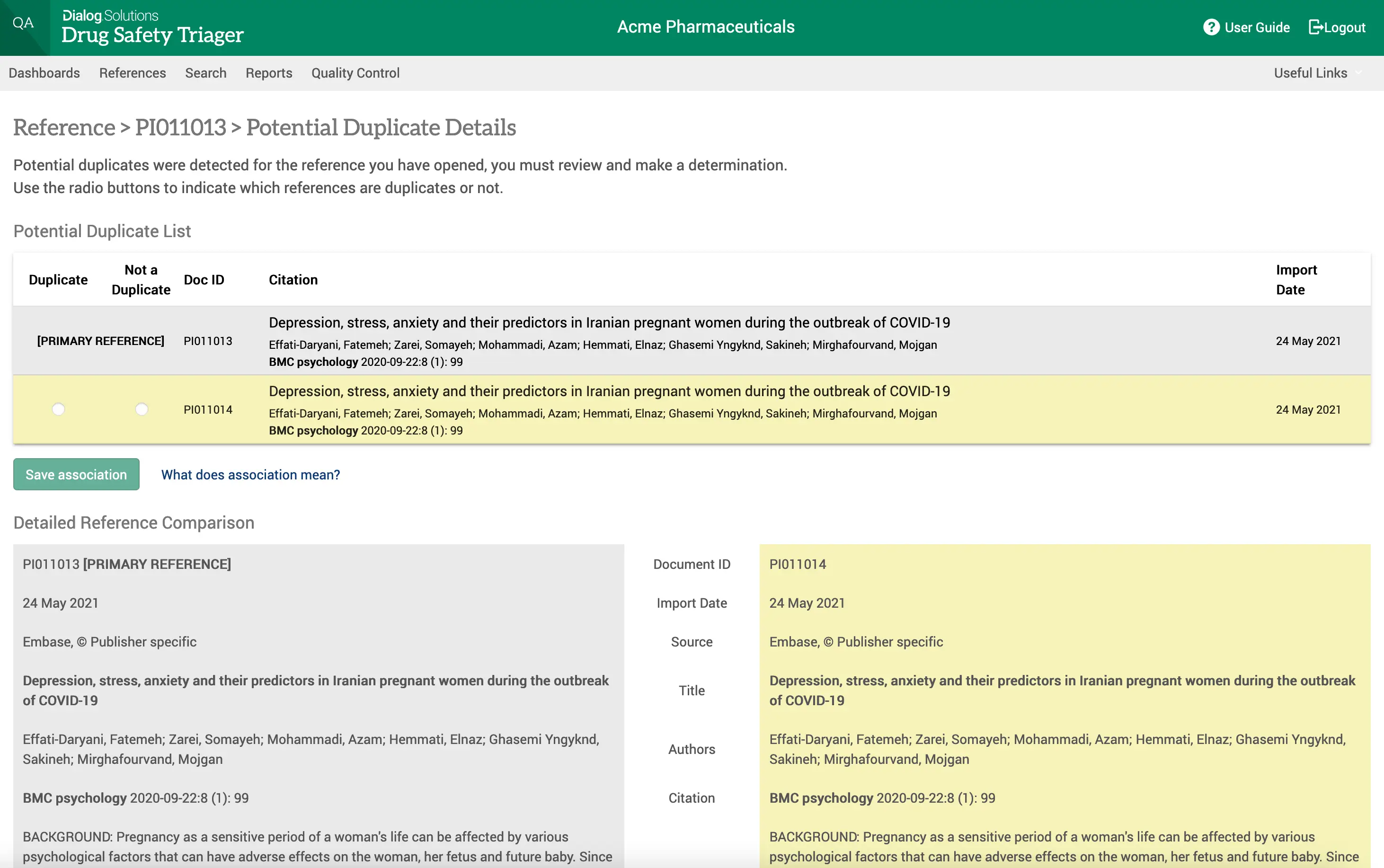
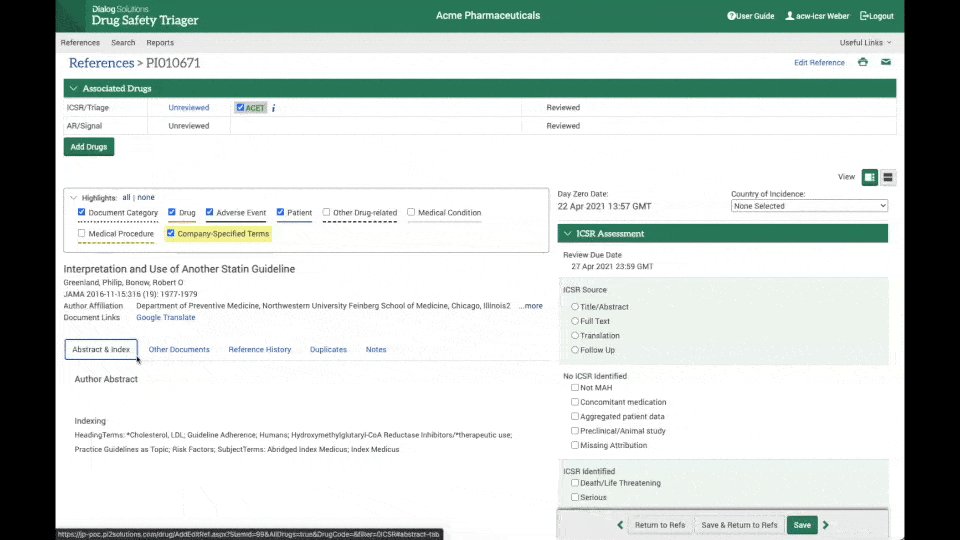
Drug Safety Triager automatically imports references from Dialog and other literature providers. The system auto-deduplicates at import and consolidates references retrieved for multiple drugs. Additional manual deduplication allows users to further reduce articles that need to be reviewed.
An automated workflow ensures references can be reviewed step-by step by multiple users for multiple purposes in an organised and transparent way. Automated work allocation and advancement of articles with step validation checks along the process enable all users to work independently and with full accountability.
Full oversight and administrative control
Drug Safety Triager features a range of configurable dashboards that help increase oversight of even the largest drug safety monitoring workflows. The dashboard cards provide quick access to targeted statistics for an immediate view of work status, including potential risks, priorities and longer-term trends in the review workflow. Easy to use, configurable features allow the administrator role to be performed by either a business or an IT professional.
Administrator self-serve features, including:
- Self-setup and configuration by the administrator
- Full control over account creation and user access
- Admin changes audit trail
- Ad-hoc importing of additional literature outside of scheduled alerts
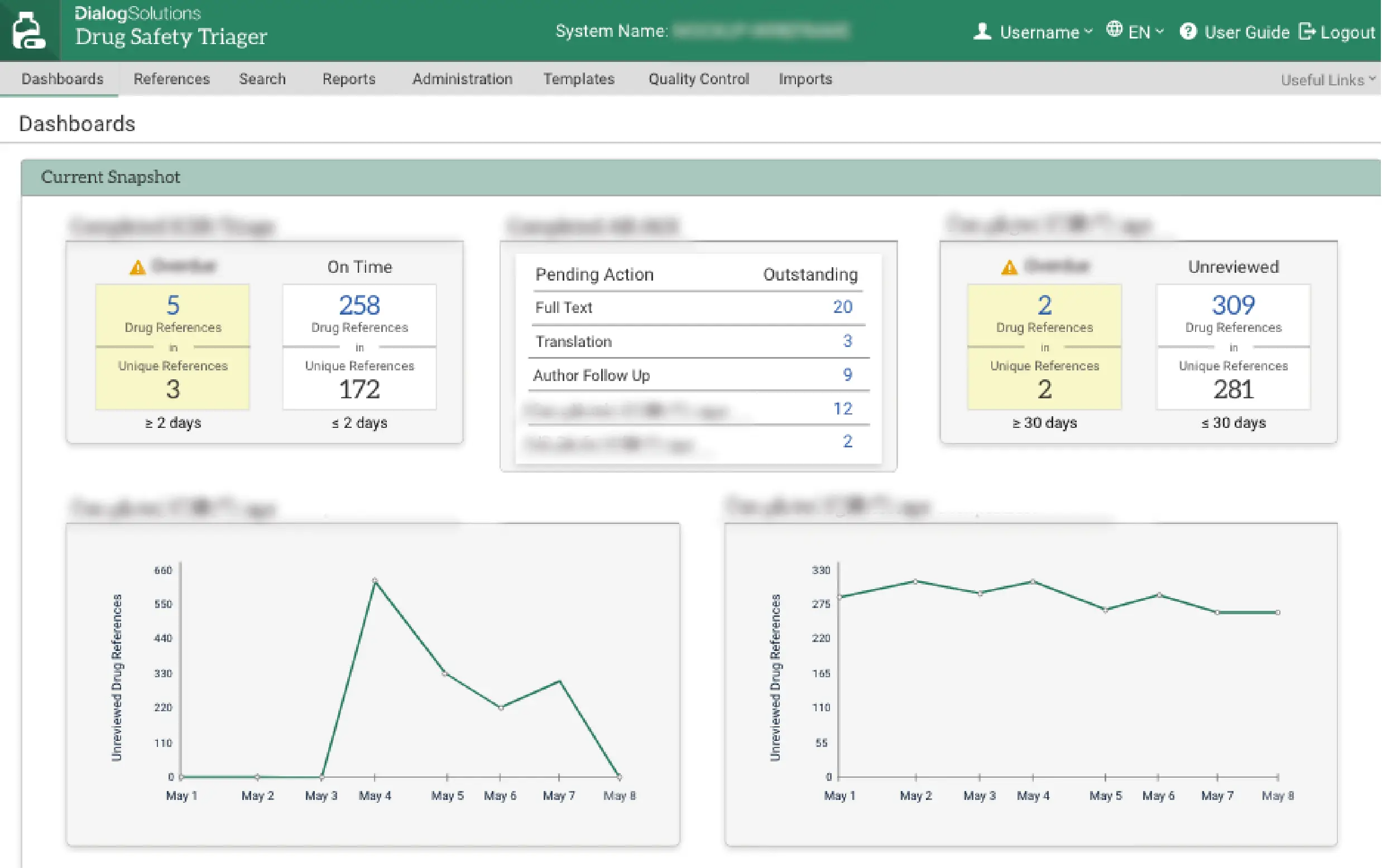
Efficiency and cost-savings
Drug Safety Triager is proven to deliver measurable efficiencies and cost savings through:
- Deduplication
- Multi-drug review
- Bulk review
- Easily accessible SOP links and supporting documents
- An intuitive and streamlined interface
The best-practice workflow reduces irrelevant literature references to be reviewed for Aggregate Reporting and Signal detection by up to 70%.
GxP compliant pharmacovigilance (PV) literature review software
Dialog Solutions’ Quality Management System provides the framework to consistently deliver products and services that meet customer and regulatory requirements. We are ISO 9001:2015 certified within the scope of Medical Literature Monitoring Solutions.
Each implementation of Drug Safety Triager is delivered with a validation package. The validation process and associated documentation are in line with pharmaceutical industry and health authority regulations.
All literature references undergo a systematic review process which automatically captures all users’ actions and adds a permanent, non-editable audit trail to the record. This process ensures audit readiness for any organisation using Drug Safety Triager.

Instant access to full-text articles
Through the integration between Drug Safety Triager and Article Galaxy from Reprints Desk, full text acquisition is completely seamless. Literature reviewers can instantly access the full-text article needed for review, quickly upload it to the reference and update the day 0 date, further increasing efficiency and reducing the chance of errors.
