As you might already know, Drug Safety Triager is our pharmacovigilance literature screening solution, designed to help pharmaceutical companies meet their regulatory requirements. It is already used by 6 of the top 10 global pharmaceutical companies, as well as smaller pharmas, biotechs and Contract Research Organisations (CROs) to monitor medical and scientific literature for Individual Case Safety Reporting (ICSR), Aggregate Reports and Signal detection. But there’s always more that can be done, and this is why we’re announcing the launch of the next-generation of pharmacovigilance literature monitoring with Drug Safety Triager.
Building on our previous successes, the latest evolution of Drug Safety Triager takes drug safety literature monitoring to a new level of efficiency, compliance and cost-effectiveness.
GxP Compliant Literature Monitoring
Each implementation of Drug Safety Triager is delivered with a validation package to ensure compliance to health authority expectations.
Drug Safety Triager also automatically captures all users’ actions and appends a permanent, non-editable audit trail to the record for all literature references related to medicinal product safety. This process ensures the audit readiness of all records at any time.
Cost-effective literature reviewing
The next-generation pharmacovigilance literature monitoring with Drug Safety Triager adds intelligent automation to the range of automated steps and controls currently enjoyed by our customers. When Drug Safety Triager is used with DialogML, our AI for pharmacovigilance literature monitoring, literature review teams can find ICSRs 5x faster and halve the volume of literature to be reviewed for ICSRs without introducing additional risk.
Configurable software
The next-generation Drug Safety Triager has an intuitive, configurable interface offering a range of user-friendly features to further enhance pharmacovigilance literature monitoring productivity, including:
- Automatic import with automatic work allocation assures that all references are received and reviewed
- Enhanced reference deduplication that eliminates the waste of reviewing same references multiple times
- Workflow for global team and global GVP regulations allowing you to make the most of your resources
- Advanced supporting documents management
- Compliant output: ICSR E2B R3 output to case processing, Aggregate Reports in Vancouver style and Safety Signals in line listing.
- Enhanced status reports for proactive operation monitoring, vendor oversight, reconciliation, performance notifications
Improved oversight
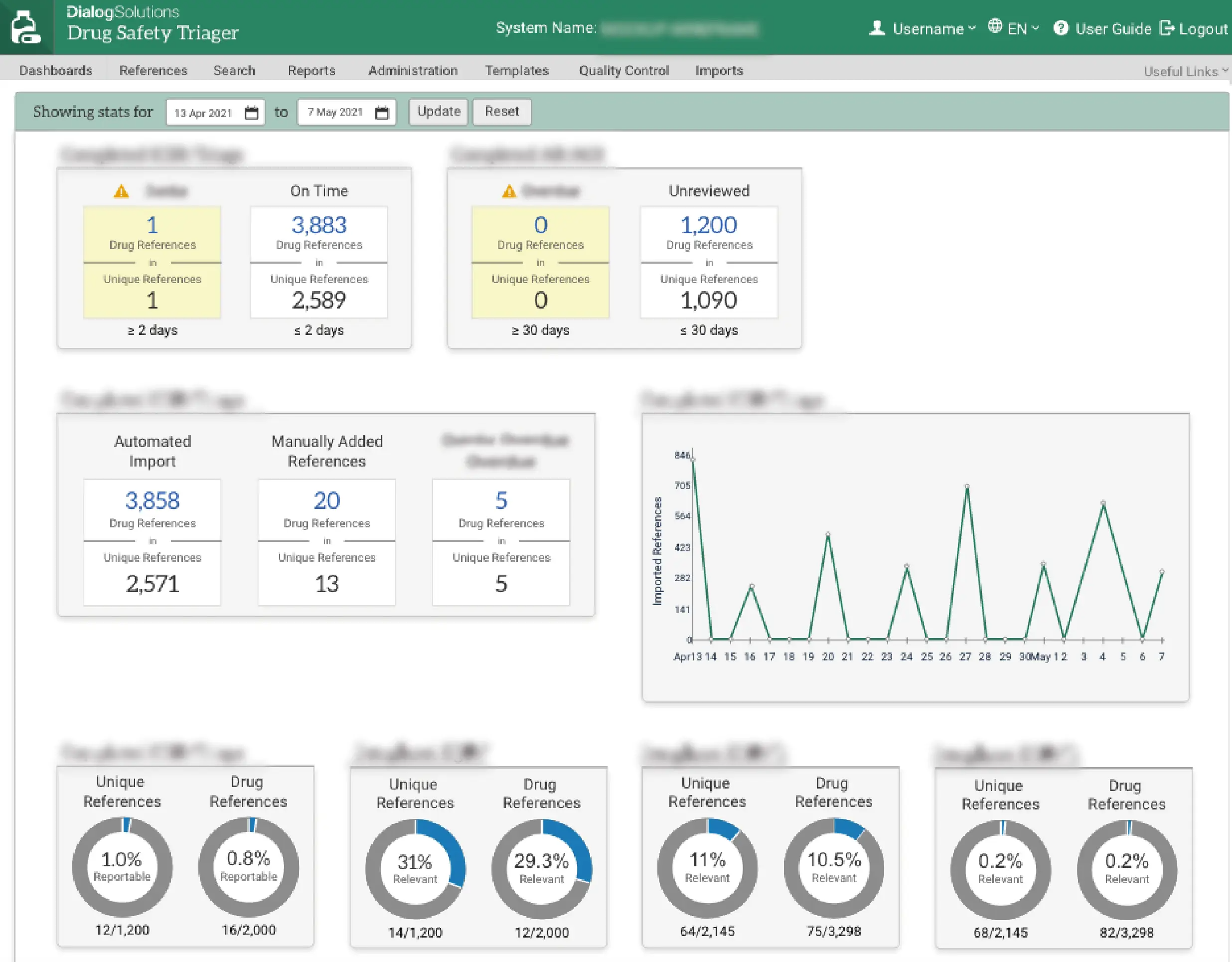
Drug Safety Triager features a range of configurable dashboards providing an at a glance view of the status of the medical literature monitoring workflow, including potential risks, priorities and longer-term trends.
Full administrative control
The next-generation Drug Safety Triager can be used by organisations of any size, from small biopharmas to large multi-national pharmaceuticals. It includes a number of self-serve features such as
- Self-setup and configuration by the administrator
- Full control over account creation and user access
- Admin changes audit trail
- Ad-hoc importing of additional literature outside of scheduled alerts
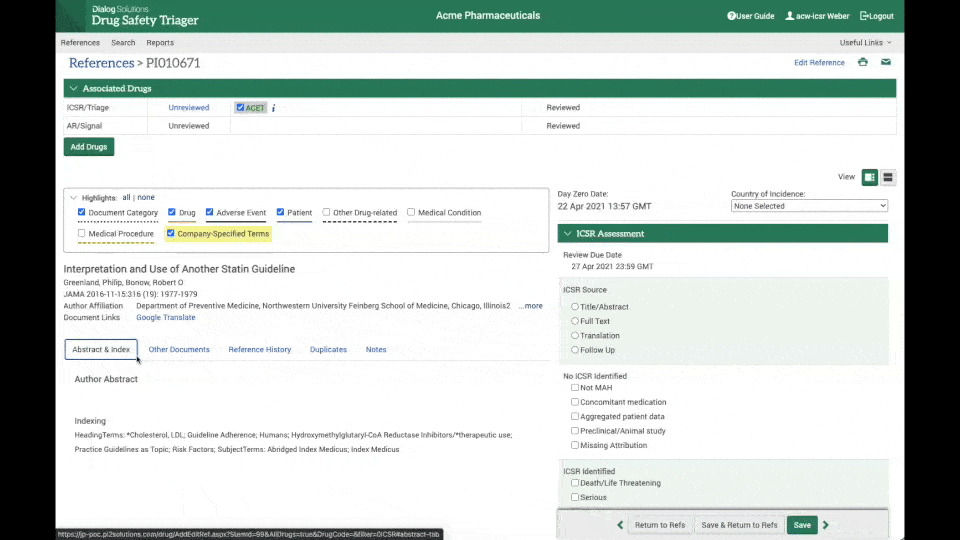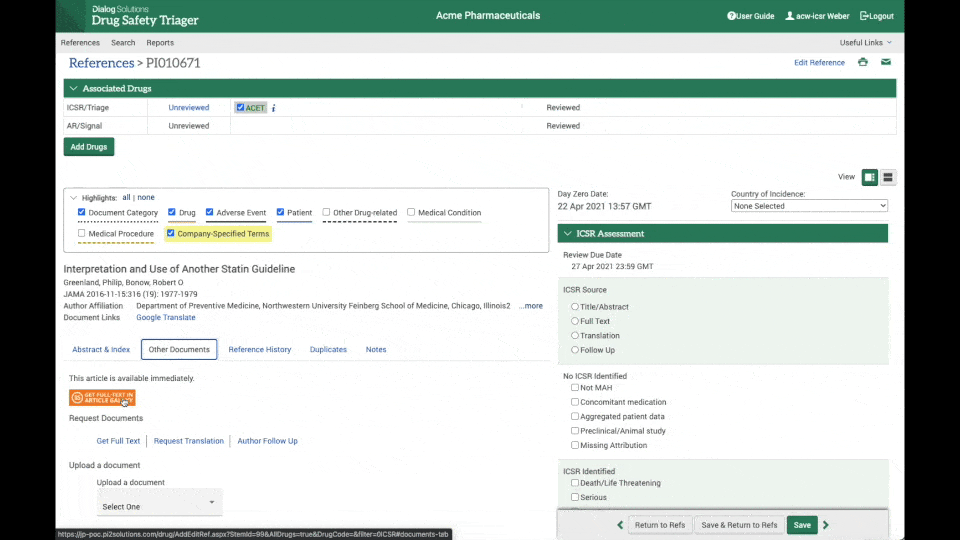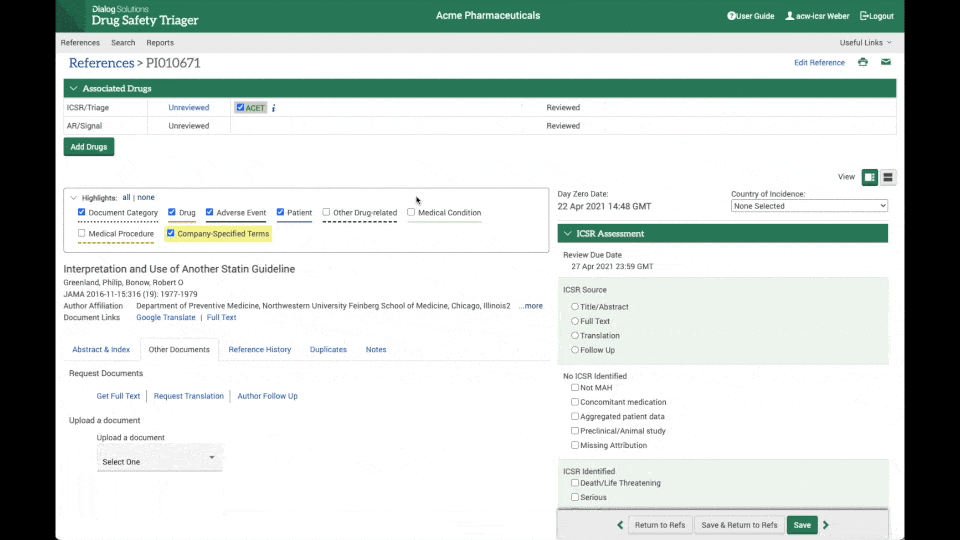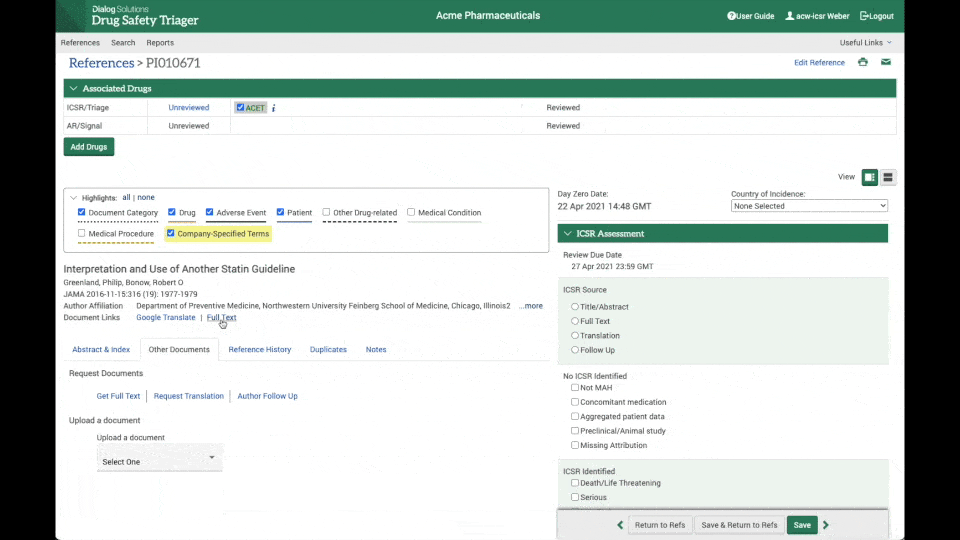
Instant access to full-text articles
Through the integration between Drug Safety Triager and Article Galaxy from Reprints Desk, full-text acquisition is made seamless.
Literature reviewers can instantly access the full-text article needed for review, upload it to the reference in a matter of seconds. and update day 0 date, further increasing efficiency and reducing the chance of errors. (see the clip on the right).
What’s next?
This is just a quick overview of what the next-generation pharmacovigilance literature monitoring solution has to offer. Take a look at the Drug Safety Triager product page for an in-depth look at all the features, or see how it fits into our end-to-end pharmacovigilance literature monitoring workflow.
Or if you’d like to discuss your literature monitoring process or see a demo of the platform, get in touch with us to arrange a call.





