At Dialog Solutions, we work with some of the world’s biggest pharmaceutical companies to deliver modular, end-to-end medical literature monitoring for pharmacovigilance. What’s more, we’re the only organisation that offers a truly end-to-end approach to drug safety literature monitoring.
We provide the content (including the top biopharma collections), software (Dialog with Alerts Management Drug Safety Triager and PinPoint) and services (our own in-house literature review teams) to deliver a complete medical literature monitoring workflow.
In a nutshell, Dialog is our search platform, providing anyone with the ability to precisely search over 140 content databases. Dialog Alerts Manager allows you to create automated search alerts (and manage your search strategies in one place). Drug Safety Triager is our fully validated and audit-ready literature monitoring software tool. And PinPoint is our product literature database. Combined with our literature review teams, this is our end-to-end pharmacovigilance literature monitoring solution:
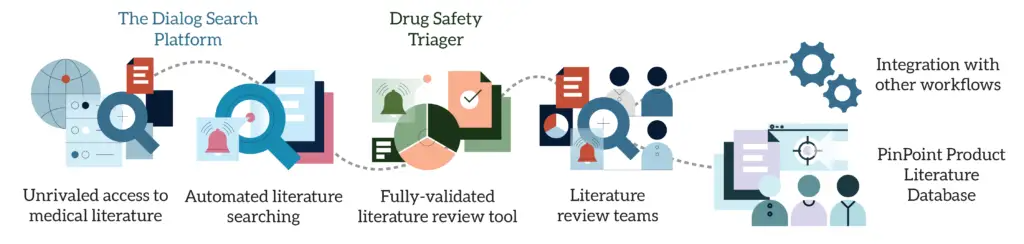
But if you’re tasked with improving your approach to pharmacovigilance literature monitoring, you’ll want to understand exactly how we’re able to deliver a truly end-to-end workflow.
That’s why we’ve created a series of videos that demonstrate how Dialog, Alerts Manager and Drug Safety Triager work together in our end-to-end approach. In the playlist below, we’ll show you:
- How to create a search for adverse effects on Dialog. You’ll learn how to structure your searches, choose databases and take advantage of the precision search features of Dialog
- Why Dialog Alerts Manager can make your life easier, helping you to set up and update your search strategies for adverse events monitoring, and seamlessly deliver the articles retrieved to your review team as periodic alerts
- How Dialog and Alerts Manager work with our validated literature review workflow tool Drug Safety Triager to make the literature review process more efficient and compliant





